How Long Does Rezum Treatment Last
Skip to main content Skip to search Skip to product navigation
Safe, Effective, Durable
Rezūm™ H2o Vapor Therapy is now the only in‐function BPH therapy with proven prostate volume reduction and long-term durability out to v years while preserving sexual function.1
The 5-year clinical trial, conducted by Kevin McVary, MD, and Claus Roehrborn, Medico, was published in the Periodical of Urology in September 2021.

surgical retreatment
charge per unit through 5 yearsone

medication retreatment
rate through 5 yearsane

average
process time1
"Rezūm Therapy has been embraced by urology practices, health engineering cess bodies, and urologic societies throughout the US and Europe. This adoption is attributed to the evidence of its clinical advantages, including sustained relief of LUTS, enhanced quality of life, and durability of handling response."1
Lasting Relief for Patients
BPH can disrupt patients' lives. Our goal is to provide relief that lasts, with a low surgical retreatment rate while minimizing complications.
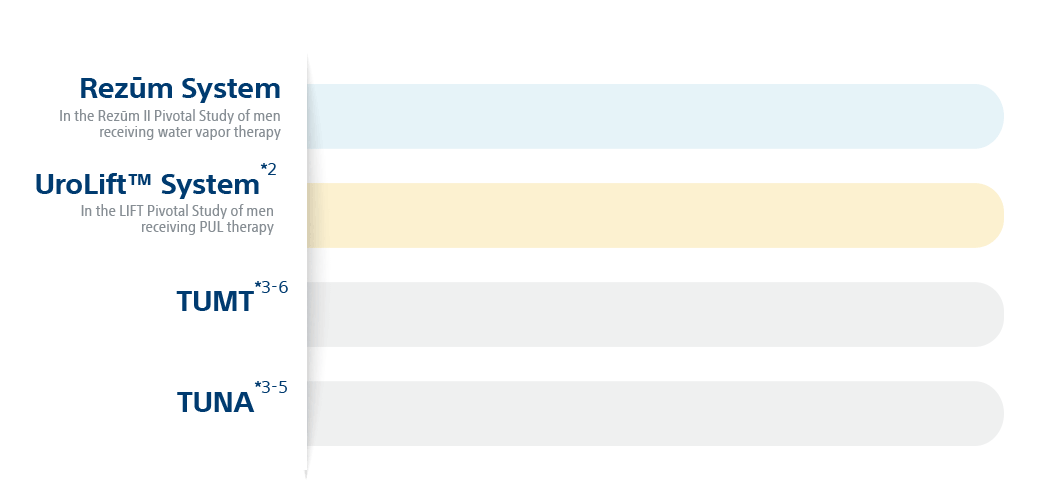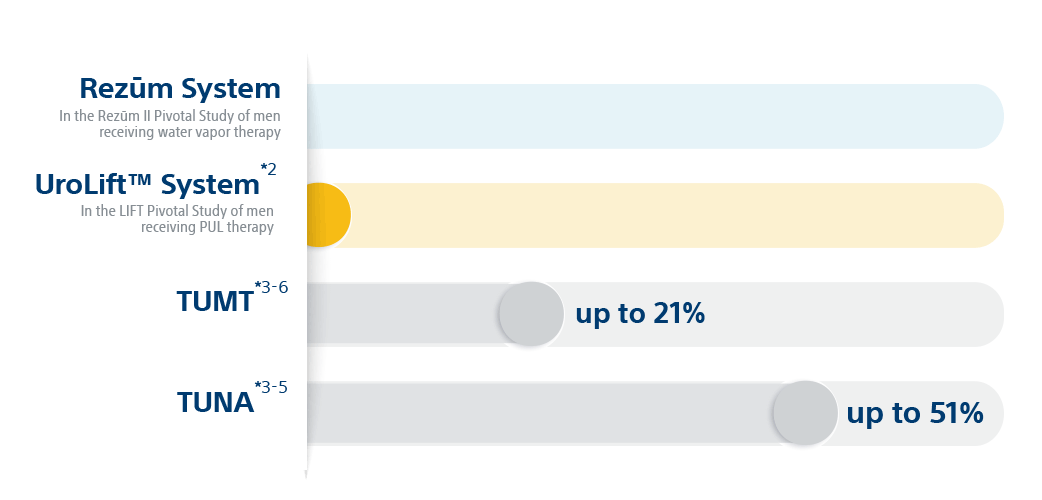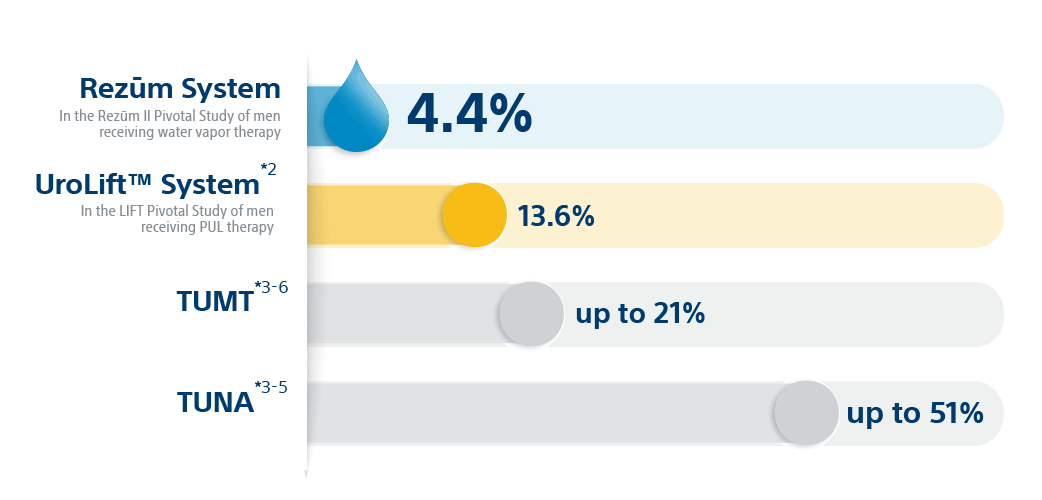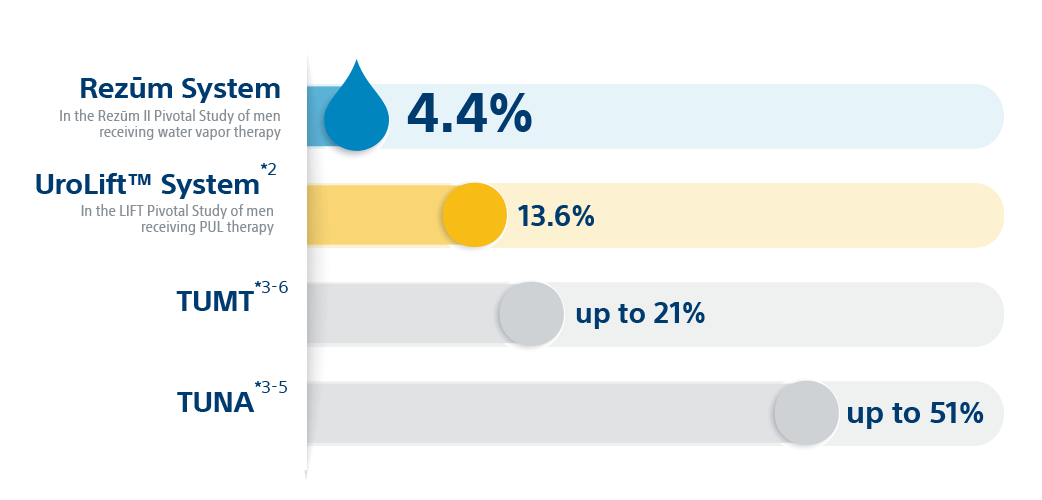
*Results from unlike clinical investigations are not directly comparable. Data provided for educational purposes just.
Through 5 years of follow-upwards, patients experienced significant improvements to their BPH symptoms.1
Convective steam therapy is proven to reduce prostate volume, without thermal lesions occurring exterior of the treatment zone.seven Unlike other MISTs, Rezūm™ Therapy is recommended by the AUA for treating center lobe obstruction (MLO).8 And the 5-yr written report affirms a best-in-course surgical retreatment rate of just iv.four%.1
That'southward why patients are satisfied with Rezūm Therapy. According to a 4-year single-center study of 255 men, 97% would recommend this treatment to a friend.9
The Results
The Rezūm pivotal report supports that Rezūm Therapy is a safe and effective in-office BPH treatment that preserves sexual office out to five years, and provides significant, sustained comeback of lower urinary tract symptoms (LUTS) and quality of life for patients suffering from BPH.1
Study Details
Overview
This was a multicenter (xv sites), randomized, sham-controlled trial of Rezūm H2o Vapor Therapy in men with moderate-to-severe LUTS due to benign prostatic hyperplasia (BPH), with the inclusion of the final surgical and BPH medication retreatment rates. Thermal therapy involved injection of water vapor, using the Rezūm Organization, into obstructive tissue, including the middle lobe and/or enlarged central zone.
Patient Profile
197 subjects ≥ 50 years old
| International Prostate Symptom Score (IPSS) | Maximum flow rate (Qmax) | Prostate volume |
| ≥xiii | ≤ 15 ml/s | thirty-80 cc |
Patients were randomized two:1
(Thermal therapy with the Rezūm Arrangement: sham command rigid cystoscopy)
All Rezūm Therapies were completed in the office or convalescent-surgery heart.
Preserve Sexual Role with Rezūm Therapy
Sexual office can be important to patients. Rezūm Therapy patients experience durable LUTS relief while preserving sexual office.ane
Results from a mail-hoc assay of the v-yr Rezūm Ii prospective, multi-center, randomized, blinded controlled trial suggests Rezūm Therapy does not cause clinically meaningful* declines in erectile function or ejaculatory office out to 5 years.ten This analysis was performed on all treated subjects who were sexually agile at baseline with no other surgical or medical management for BPH during the 5-year study menstruum.
* None of the patients in the written report changed sexual function category (no dysfunction, balmy, moderate, severe).
Leading Urologist Kevin McVary, Medico, FACS, presents the concluding outcomes of the Rezūm H2o Vapor five-year pivotal study.
Request More than Information
Sign up to learn more than about Rezūm Water Vapor Therapy.
About Rezūm Therapy
Rezūm Water Vapor Therapy uses convective energy to remove obstructive prostate tissue, treating the cause of BPH. This handling delivers targeted, controlled doses of the stored thermal energy in water vapor straight to the region of the prostate gland with the obstructive tissue causing the lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH).11
More Information
Related Content



Indications, Safety and Warnings
References
- McVary KT, Gittelman MC, Goldberg KA, et al. Final five-twelvemonth outcomes of the multicenter randomized sham-controlled trial of Rezūm h2o vapor thermal therapy for treatment of moderate-to-severe lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2021 Sep;206(3):715-24.
- Roehrborn CG, Barkin J, Gange SN, et al. Five-year results of the prospective randomized controlled prostatic urethral L.I.F.T. study. Tin can J Urol. 2017 Jun;24(3):8802-13.
- Zlotta AR, Giannakopoulos X, Maehlum O, et al. Long-term evaluation of transurethral needle ablation of the prostate (TUNA) for treatment of symptomatic beneficial prostatic hyperplasia: clinical outcome up to five years from three centers.Eur Urol. 2003 Jul;44(ane):89-93.
- Rosario DJ, Phillips JT, Chapple CR. Chapple, Durability and price-effectiveness of transurethral needle ablation of the prostate every bit an alternative to transurethral resection of the prostate when alpha-adrenergic antagonist therapy fails. J Urol. 2007 Mar;177(3):1047-51; give-and-take 1051.
- Hill B, Belville W, Bruskewitz R, et al. Transurethral needle ablation versus transurethral resection of the prostate for the treatment of symptomatic benign prostatic hyperplasia: 5-twelvemonth results of a prospective, randomized, multicenter clinical trial.J Urol. 2004 Jun;171(half dozen Pt 1):2336-xl.
- Miller PD, Kastner C, Ramsey EW, et al. Cooled thermotherapy for the treatment of benign prostatic hyperplasia: durability of results obtained with the Targis system. Urology. 2003 Jun;61(6):1160-4.
- Mynderse LA, Hanson D, Robb RA, et al. Rezūm System water vapor treatment for lower urinary tract symptoms/benign prostatic hyperplasia: validation of convective thermal energy transfer and characterization with magnetic resonance imaging and 3-dimensional renderings. Urology. 2015 Jul;86(ane):122-vii.
- Lerner LB, McVary KT, Barry MJ, et al. Direction of lower urinary tract symptoms attributed to beneficial prostatic hyperplasia: AUA Guideline 2021. J Urol. 2021 Oct;206:806-26.
- Mooney R, Goldberg Grand, Wong D, et al. Convective radio frequency thermal therapy for treatment of benign prostatic hyperplasia: Singe function experience with 255 patients over 4 years. Urol Pract. 2020 Jan;7(1):28-33.
- McVary KT, El-Arabi A, Roehrborn C. Preservation of Sexual Function 5 Years After Water Vapor Thermal Therapy for Benign Prostatic Hyperplasia. Sexual practice Med. 2021 Dec;ix(6):100454.
- Data on File with Boston Scientific.
The Rezūm System is intended to relieve symptoms, obstructions, and reduce prostate tissue associated with BPH. It is indicated for men ≥ fifty years of age with a prostate volume 30cm3 ≤ 80cmiii. The Rezūm Organization is likewise indicated for handling of prostate with hyperplasia of the key zone and/or a median lobe. Potential risks associated with Rezūm Water Vapor Therapy include but are not limited to dysuria, hematuria, hematospermia, decrease in ejaculatory volume, suspected urinary tract infection (UTI), urinary frequency, and retention or urgency.
Caution: U.S. Federal police restricts this device to sale past or on the order of a dr..
Important Data: These materials are intended to describe common clinical considerations and procedural steps for the use of referenced technologies but may not exist appropriate for every patient or case. Decisions surrounding patient intendance depend on the medico's professional judgment in consideration of all available information for the individual case. Boston Scientific (BSC) does not promote or encourage the use of its devices exterior their canonical labeling. Case studies are not necessarily representative of clinical outcomes in all cases equally individual results may vary.
All images are the property of Boston Scientific. All trademarks are the holding of their respective owners.
How Long Does Rezum Treatment Last,
Source: https://www.bostonscientific.com/en-US/medical-specialties/urology/products/rezum/rezum-5-year-data.html
Posted by: glovergrater.blogspot.com



0 Response to "How Long Does Rezum Treatment Last"
Post a Comment